Continuous Submicron Particle Separation Via Vortex-Enhanced Ionic Concentration Polarization: A Numerical Investigation
Abstract
:1. Introduction
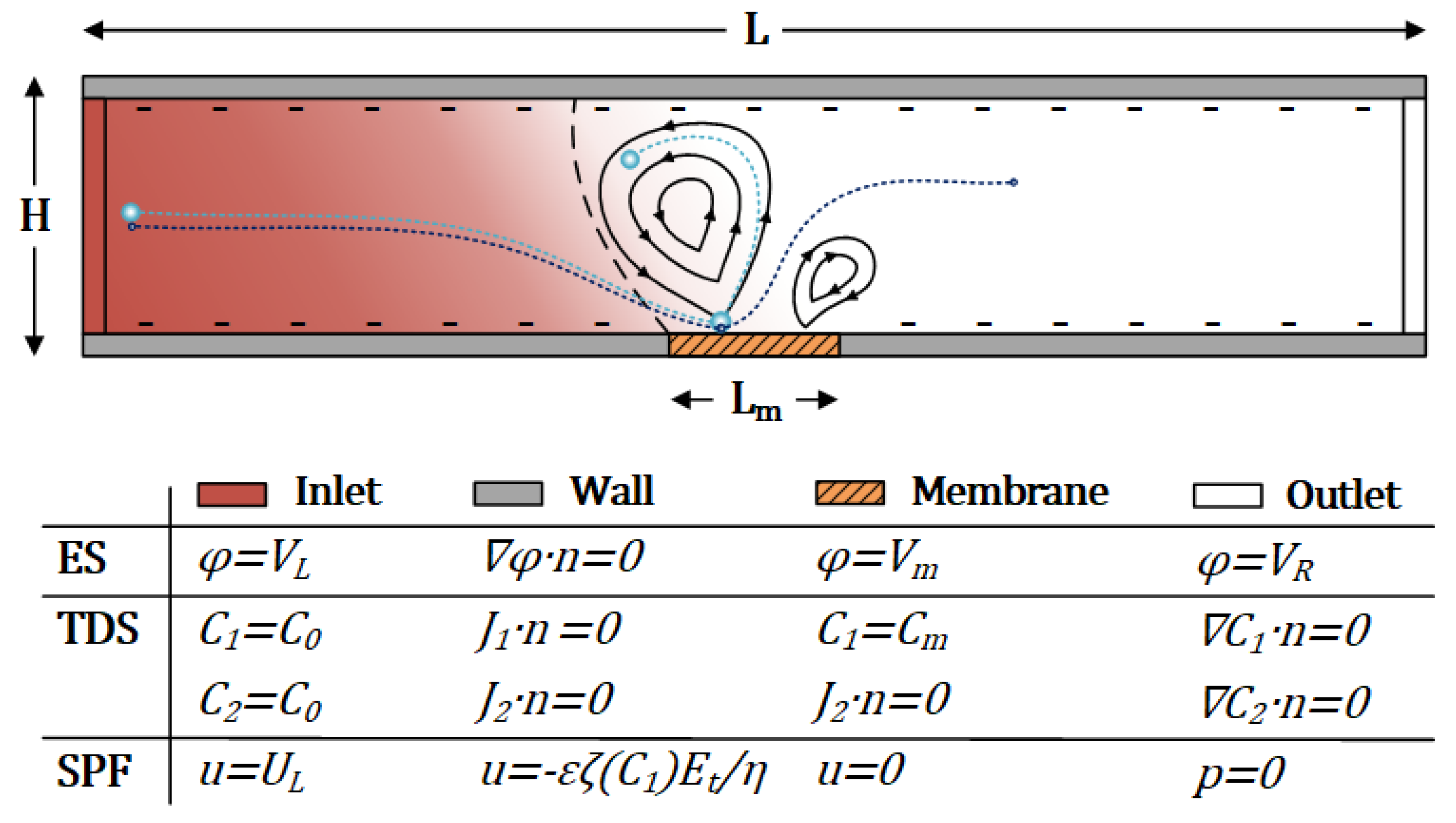
2. Device Principle and Numerical Model
2.1. Electric Field
2.2. Concentration Field
2.3. Flow Field
2.4. Particle Tracing Approaches
2.4.1. Newtonian
2.4.2. Massless
2.5. Computational Implementation
3. Result and Discussion
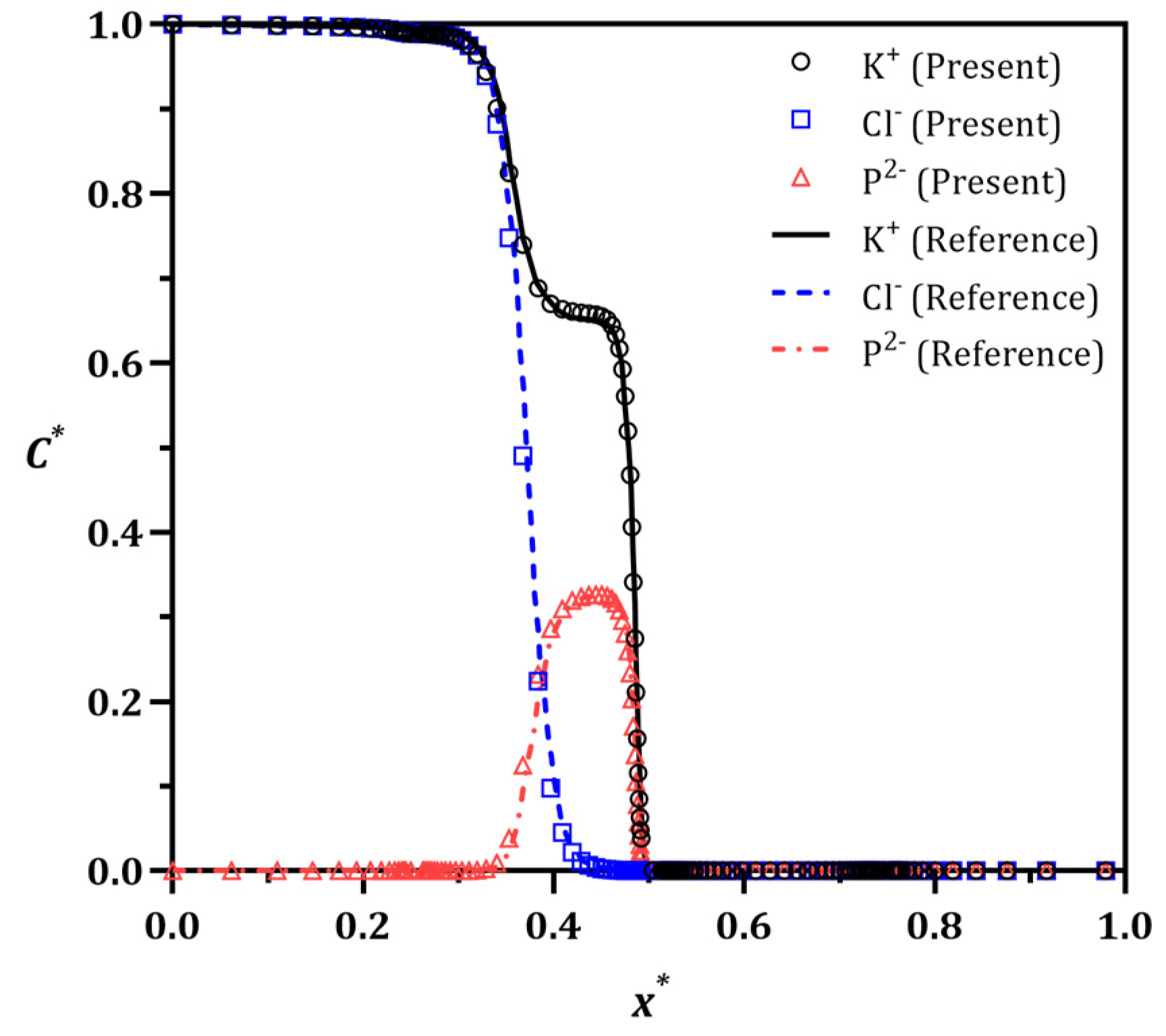
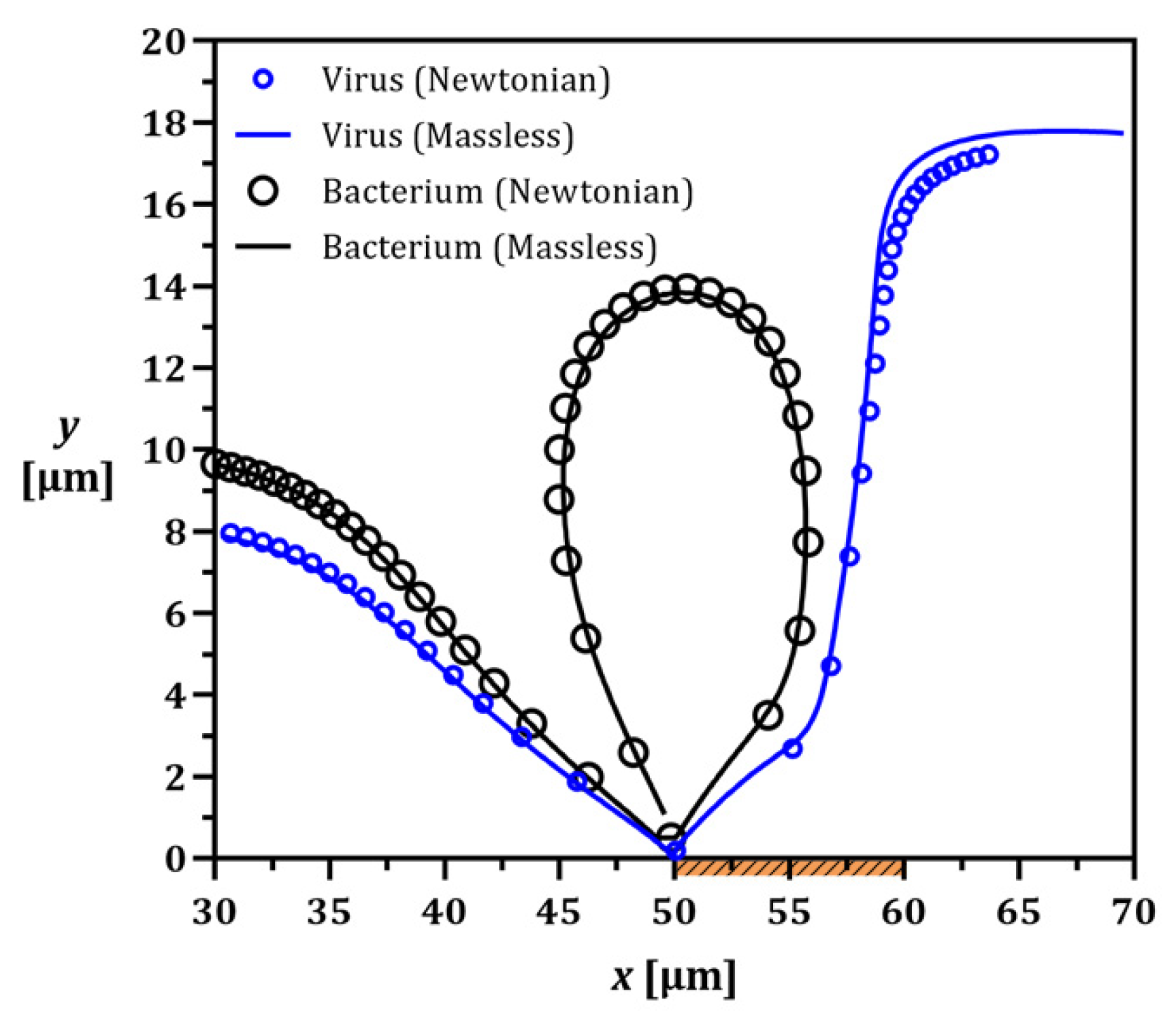
3.1. Method Validation
3.2. Mesh Sensitivity Analysis
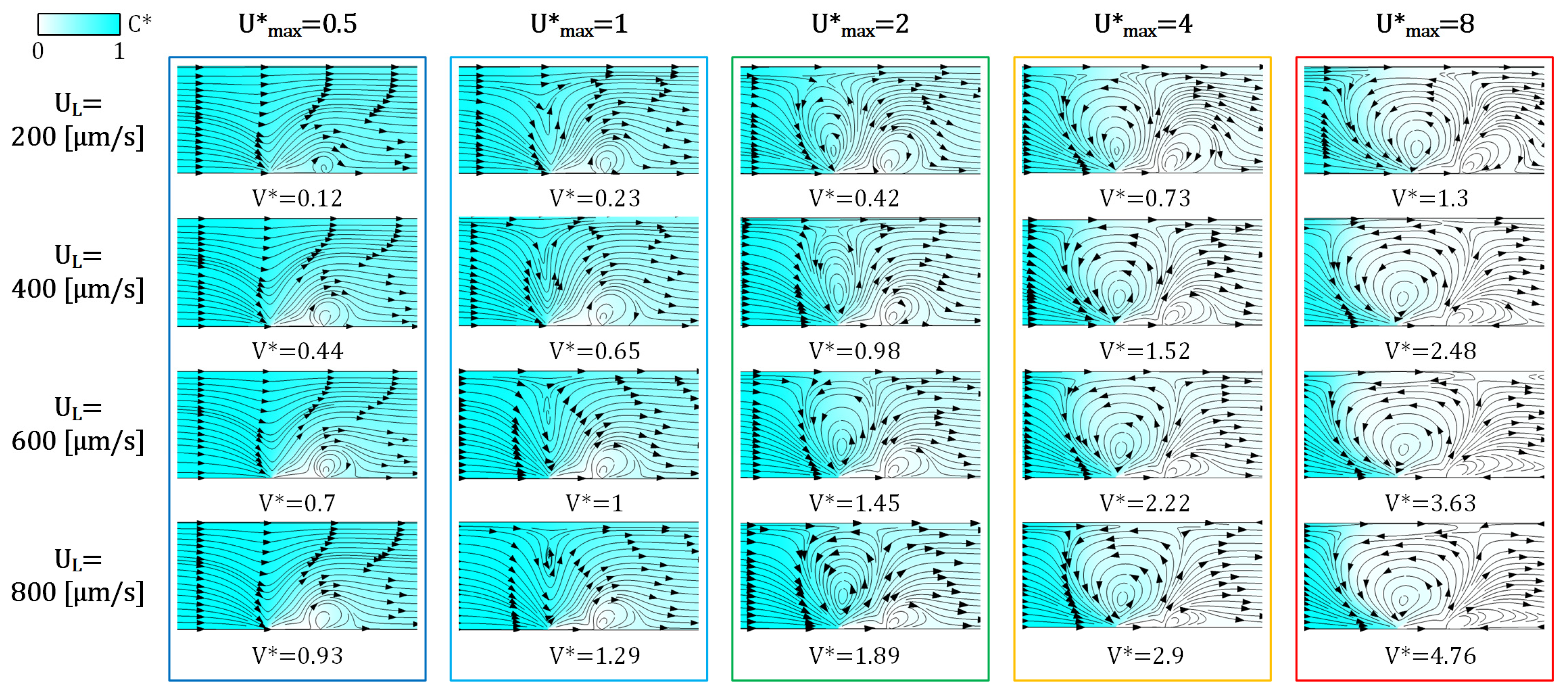
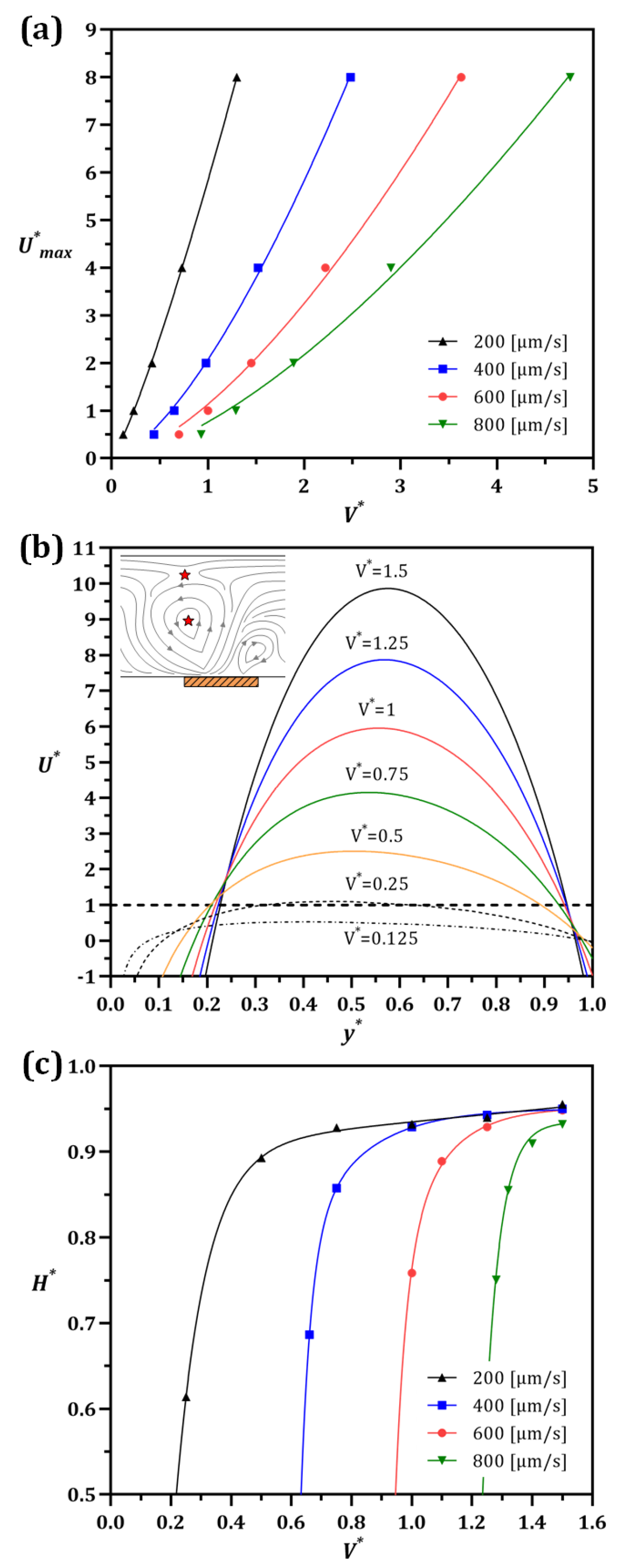
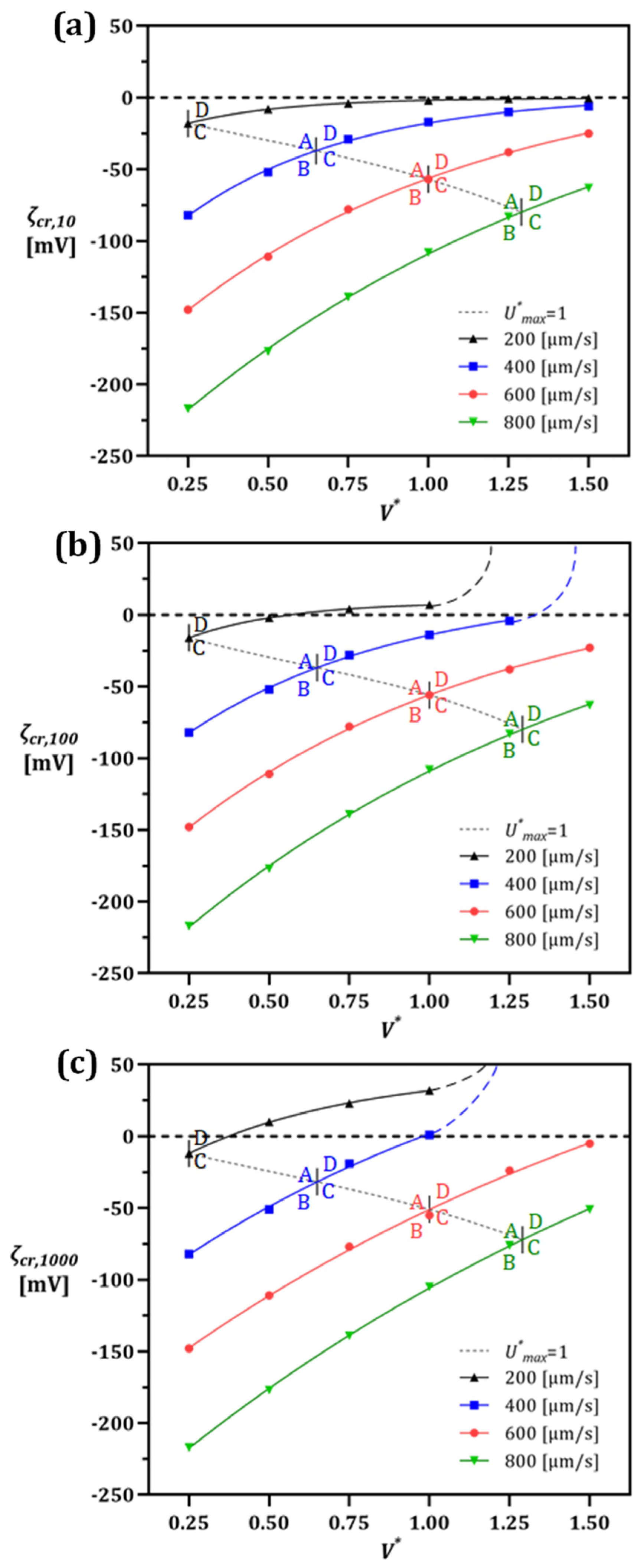
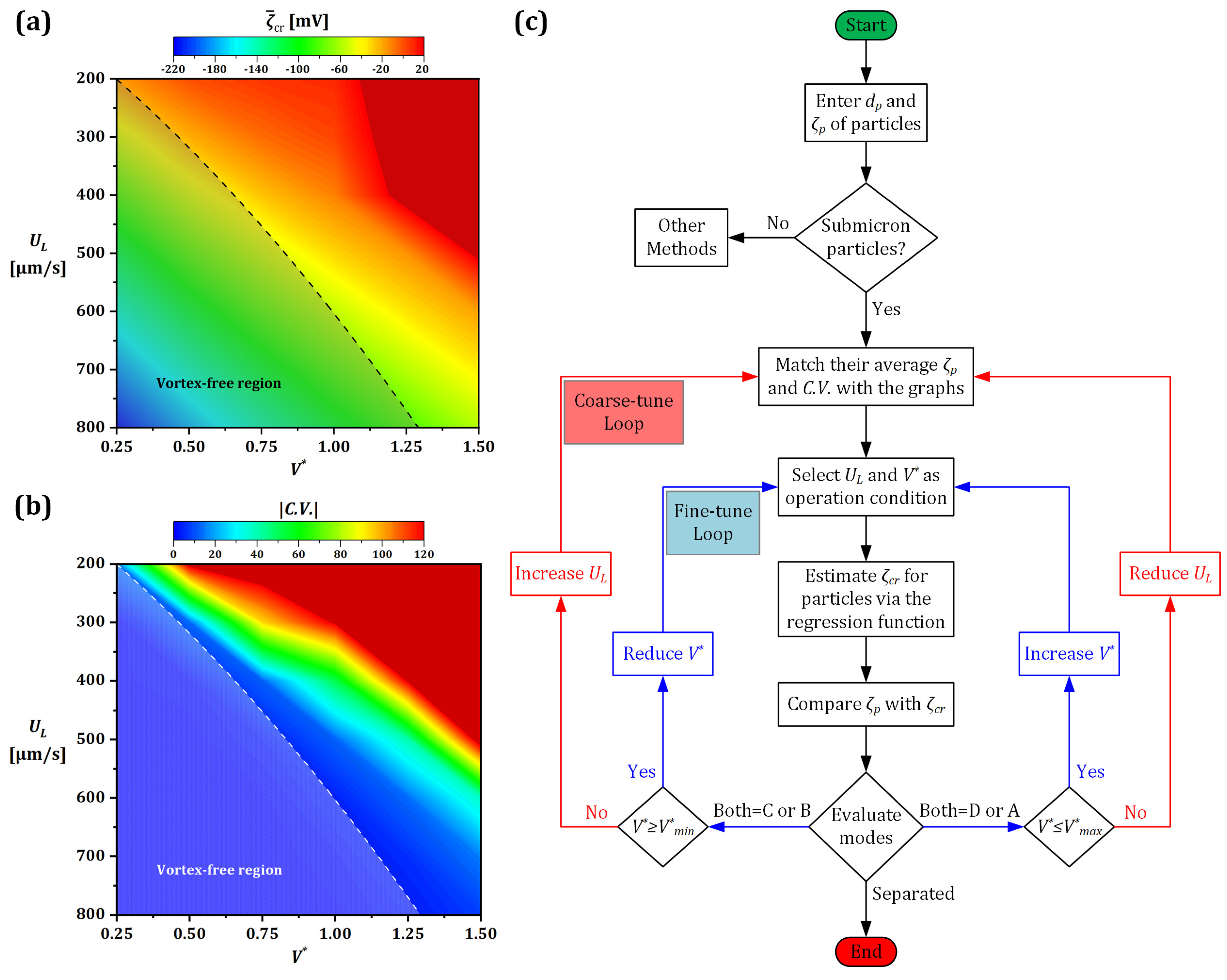
3.3. The Effect of Voltage/Flow Rate on IDZ
3.4. Newtonian vs. Massless Particle Tracing Models
3.5. Particle Separation Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S.; Kobayashi, I.; Nakajima, M.; Wu, D.; Nandagopal, S.; Lin, F. Microfluidics for food, agriculture and biosystems industries. Lab Chip 2011, 11, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.; Fallahi, H.; Dai, Y.; Yadav, S.; Hettiarachchi, S.; McNamee, A.; An, H.; Xiang, N.; Nguyen, N.T.; Zhang, J. Tuning particle inertial separation in sinusoidal channels by embedding periodic obstacle microstructures. Lab Chip 2022, 22, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
- Lapizco-Encinas, B.H.; Simmons, B.A.; Cummings, E.B.; Fintschenko, Y. Insulator-based dielectrophoresis for the selective concentration and separation of live bacteria in water. Electrophoresis 2004, 25, 1695–1704. [Google Scholar] [CrossRef]
- Roelofs, S.H.; Van Den Berg, A.; Odijk, M. Microfluidic desalination techniques and their potential applications. Lab Chip 2015, 15, 3428–3438. [Google Scholar] [CrossRef]
- Nguyen, N.-T.; Wereley, S.; Shaegh, S.A.M. Fundamentals and Applications of Microfluidics; Artech House: Norwood, MA, USA, 2019; ISBN 9788578110796. [Google Scholar]
- Abdulbari, H.A.; Basheer, E. Microfluidic Desalination: A New Era Towards Sustainable Water Resources. ChemBioEng Rev. 2021, 8, 121–133. [Google Scholar] [CrossRef]
- Kim, S.J.; Ko, S.H.; Kang, K.H.; Han, J. Direct seawater desalination by ion concentration polarization. Nat. Nanotechnol. 2010, 5, 297–301. [Google Scholar] [CrossRef]
- Fung, C.W.; Chan, S.N.; Wu, A.R. Microfluidic single-cell analysis—Toward integration and total on-chip analysis. Biomicrofluidics 2020, 14, 021502. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, X.; Wang, J.; Wang, C.; Yan, Y.; Wu, A.; Ren, Y. Public-health-driven microfluidic technologies: From separation to detection. Micromachines 2021, 12, 391. [Google Scholar] [CrossRef]
- Basiri, A.; Heidari, A.; Nadi, M.F.; Fallahy, M.T.P.; Nezamabadi, S.S.; Sedighi, M.; Saghazadeh, A.; Rezaei, N. Microfluidic devices for detection of RNA viruses. Rev. Med. Virol. 2021, 31, 1–11. [Google Scholar] [CrossRef]
- Salafi, T.; Zhang, Y.; Zhang, Y. A Review on Deterministic Lateral Displacement for Particle Separation and Detection. Nano-Micro Lett. 2019, 11, 77. [Google Scholar] [CrossRef] [Green Version]
- Antfolk, M.; Laurell, T. Continuous flow microfluidic separation and processing of rare cells and bioparticles found in blood—A review. Anal. Chim. Acta 2017, 965, 9–35. [Google Scholar] [CrossRef]
- Razavi Bazaz, S.; Mihandust, A.; Salomon, R.; Joushani, H.A.N.; Li, W.; Amiri, H.A.; Mirakhorli, F.; Zhand, S.; Shrestha, J.; Miansari, M.; et al. Zigzag microchannel for rigid inertial separation and enrichment (Z-RISE) of cells and particles. Lab Chip 2022, 22, 4093–4109. [Google Scholar] [CrossRef]
- Vatandoust, F.; Amiri, H.A.; Mas-hafi, S. DLDNN: Deterministic Lateral Displacement Design Automation by Neural Networks. arXiv 2022, arXiv:2208.14303. [Google Scholar]
- Collins, D.J.; Alan, T.; Neild, A. Particle separation using virtual deterministic lateral displacement (vDLD). Lab Chip 2014, 14, 1595–1603. [Google Scholar] [CrossRef]
- Collins, D.J.; Khoo, B.L.; Ma, Z.; Winkler, A.; Weser, R.; Schmidt, H.; Han, J.; Ai, Y. Selective particle and cell capture in a continuous flow using micro-vortex acoustic streaming. Lab Chip 2017, 17, 1769–1777. [Google Scholar] [CrossRef]
- Ke, L.; Yang, D.; Gao, G.; Wang, H.; Yu, Z.; Rao, P.; Zhou, J.; Wang, Q. Rapid separation and quantification of self-assembled nanoparticles from a liquid food system by capillary zone electrophoresis. Food Chem. 2020, 319, 126579. [Google Scholar] [CrossRef]
- Kwizera, E.A.; Sun, M.; White, A.M.; Li, J.; He, X. Methods of Generating Dielectrophoretic Force for Microfluidic Manipulation of Bioparticles. ACS Biomater. Sci. Eng. 2021, 7, 2043–2063. [Google Scholar] [CrossRef]
- Outokesh, M.; Amiri, H.A.; Miansari, M. Numerical insights into magnetic particle enrichment and separation in an integrated droplet microfluidic system. Chem. Eng. Process.—Process Intensif. 2022, 170, 108696. [Google Scholar] [CrossRef]
- Li, D. Electrokinetics in Microfluidics; Elsevier: Burlington, MA, USA, 2004; ISBN 0120884445. [Google Scholar]
- Nagai, M.; Kato, K.; Soga, S.; Santra, T.S.; Shibata, T. Scalable parallel manipulation of single cells using micronozzle array integrated with bidirectional electrokinetic pumps. Micromachines 2020, 11, 442. [Google Scholar] [CrossRef] [PubMed]
- Avenas, Q.; Moreau, J.; Costella, M.; Maalaoui, A.; Souifi, A.; Charette, P.; Marchalot, J.; Frénéa-Robin, M.; Canva, M. Performance improvement of plasmonic sensors using a combination of AC electrokinetic effects for (bio)target capture. Electrophoresis 2019, 40, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Meinhart, A.D.; Ballus, C.A.; Bruns, R.E.; Pallone, J.A.L.; Godoy, H.T. Chemometrics optimization of carbohydrate separations in six food matrices by micellar electrokinetic chromatography with anionic surfactant. Talanta 2011, 85, 237–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suresh, A.; Hill, G.T.; Hoenig, E.; Liu, C. Electrochemically mediated deionization: A review. Mol. Syst. Des. Eng. 2021, 6, 25–51. [Google Scholar] [CrossRef]
- Su, X. Electrochemical interfaces for chemical and biomolecular separations. Curr. Opin. Colloid Interface Sci. 2020, 46, 77–93. [Google Scholar] [CrossRef]
- Höltzel, A.; Tallarek, U. Ionic conductance of nanopores in microscale analysis systems: Where microfluidics meets nanofluidics. J. Sep. Sci. 2007, 30, 1398–1419. [Google Scholar] [CrossRef]
- Kim, S.J.; Song, Y.A.; Han, J. Nanofluidic concentration devices for biomolecules utilizing ion concentration polarization: Theory, fabrication, and applications. Chem. Soc. Rev. 2010, 39, 912–922. [Google Scholar] [CrossRef] [Green Version]
- Son, S.Y.; Lee, S.; Lee, H.; Kim, S.J. Engineered nanofluidic preconcentration devices by ion concentration polarization. Biochip J. 2016, 10, 251–261. [Google Scholar] [CrossRef]
- Pu, Q.; Yun, J.; Temkin, H.; Liu, S. Ion-enrichment and ion-depletion effect of nanochannel structures. Nano Lett. 2004, 4, 1099–1103. [Google Scholar] [CrossRef]
- Song, S.; Singh, A.K.; Kirby, B.J. Electrophoretic concentration of proteins at laser-patterned nanoporous membranes in microchips. Anal. Chem. 2004, 76, 4589–4592. [Google Scholar] [CrossRef]
- Sung, J.K.; Han, J. Self-sealed vertical polymeric nanoporous-junctions for high-throughput nanofluidic applications. Anal. Chem. 2008, 80, 3507–3511. [Google Scholar] [CrossRef]
- Ko, S.H.; Song, Y.A.; Kim, S.J.; Kim, M.; Han, J.; Kang, K.H. Nanofluidic preconcentration device in a straight microchannel using ion concentration polarization. Lab Chip 2012, 12, 4472–4482. [Google Scholar] [CrossRef]
- Kim, M.; Jia, M.; Kim, T. Ion concentration polarization in a single and open microchannel induced by a surface-patterned perm-selective film. Analyst 2013, 138, 1370–1378. [Google Scholar] [CrossRef] [Green Version]
- Oh, Y.; Lee, H.; Son, S.Y.; Kim, S.J.; Kim, P. Capillarity ion concentration polarization for spontaneous biomolecular preconcentration mechanism. Biomicrofluidics 2016, 10, 014102. [Google Scholar] [CrossRef] [Green Version]
- Kwak, R.; Kang, J.Y.; Kim, T.S. Spatiotemporally Defining Biomolecule Preconcentration by Merging Ion Concentration Polarization. Anal. Chem. 2016, 88, 988–996. [Google Scholar] [CrossRef]
- Han, S.I.; Hwang, K.S.; Kwak, R.; Lee, J.H. Microfluidic paper-based biomolecule preconcentrator based on ion concentration polarization. Lab Chip 2016, 16, 2219–2227. [Google Scholar] [CrossRef]
- Lu, B.; Maharbiz, M.M. Ion concentration polarization (ICP) of proteins at silicon micropillar nanogaps. PLoS ONE 2019, 14, e0223732. [Google Scholar] [CrossRef]
- Han, S.I.; Yoo, Y.K.; Lee, J.J.H.; Kim, C.; Lee, K.; Lee, T.H.; Kim, H.; Yoon, D.S.; Hwang, K.S.; Kwak, R.; et al. High-ionic-strength pre-concentration via ion concentration polarization for blood-based biofluids. Sens. Actuators B Chem. 2018, 268, 485–493. [Google Scholar] [CrossRef]
- Song, H.; Wang, Y.; Garson, C.; Pant, K. Concurrent DNA preconcentration and separation in bipolar electrode-based microfluidic device. Anal. Methods 2015, 7, 1273–1279. [Google Scholar] [CrossRef] [Green Version]
- Gong, M.M.; Nosrati, R.; San Gabriel, M.C.; Zini, A.; Sinton, D. Direct DNA Analysis with Paper-Based Ion Concentration Polarization. J. Am. Chem. Soc. 2015, 137, 13913–13919. [Google Scholar] [CrossRef]
- Baek, S.; Choi, J.; Son, S.Y.; Kim, J.; Hong, S.; Kim, H.C.; Chae, J.H.; Lee, H.; Kim, S.J. Dynamics of driftless preconcentration using ion concentration polarization leveraged by convection and diffusion. Lab Chip 2019, 19, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Mogi, K.; Hayashida, K.; Honda, A.; Yamamoto, T. Development of Virus Concentration Device by Controlling Ion Depletion Zone for Ultrasensitive Virus Sensing. Electron. Commun. Jpn. 2017, 100, 56–63. [Google Scholar] [CrossRef]
- Cho, Y.; Yoon, J.; Lim, D.W.; Kim, J.; Lee, J.H.; Chung, S. Ion concentration polarization for pre-concentration of biological samples without pH change. Analyst 2016, 141, 6510–6514. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.L.; Cosgrove, B.D.; Lauffenburger, D.A.; Han, J. Microfluidic concentration-enhanced cellular kinase activity assay. J. Am. Chem. Soc. 2009, 131, 10340–10341. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.; Lee, H.; Kang, K.H.; Lim, G. Ion concentration polarization-based continuous separation device using electrical repulsion in the depletion region. Sci. Rep. 2013, 3, 3483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.; Cho, Y.; Han, S.; Lim, C.S.; Lee, J.H.; Chung, S. Microfluidic in-reservoir pre-concentration using a buffer drain technique. Lab Chip 2014, 14, 2778–2782. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, Y.; Lee, J.H.; Chung, S. Tunable sheathless microfluidic focusing using ion concentration polarization. Appl. Phys. Lett. 2015, 107, 083507. [Google Scholar] [CrossRef]
- Daiguji, H.; Yang, P.; Majumdar, A. Ion Transport in Nanofluidic Channels. Nano Lett. 2004, 4, 137–142. [Google Scholar] [CrossRef]
- Daiguji, H.; Yang, P.; Szeri, A.J.; Majumdar, A. Electrochemomechanical energy conversion in nanofluidic channels. Nano Lett. 2004, 4, 2315–2321. [Google Scholar] [CrossRef]
- Jin, X.; Joseph, S.; Gatimu, E.N.; Bohn, P.W.; Aluru, N.R. Induced electrokinetic transport in micro-nanofluidic interconnect devices. Langmuir 2007, 23, 13209–13222. [Google Scholar] [CrossRef]
- Shen, M.; Yang, H.; Sivagnanam, V.; Gijs, M.A.M. Microfluidic protein preconcentrator using a microchannel-integrated nafion strip: Experiment and modeling. Anal. Chem. 2010, 82, 9989–9997. [Google Scholar] [CrossRef]
- Jia, M.; Kim, T. Multiphysics simulation of ion concentration polarization induced by nanoporous membranes in dual channel devices. Anal. Chem. 2014, 86, 7360–7367. [Google Scholar] [CrossRef]
- Qiu, B.; Gong, L.; Li, Z.; Han, J. Electrokinetic flow in the U-shaped micro-nanochannels. Theor. Appl. Mech. Lett. 2019, 9, 36–42. [Google Scholar] [CrossRef]
- Jia, M.; Kim, T. Multiphysics simulation of ion concentration polarization induced by a surface-patterned nanoporous membrane in single channel devices. Anal. Chem. 2014, 86, 10365–10372. [Google Scholar] [CrossRef]
- Kim, J.; Cho, I.; Lee, H.; Kim, S.J. Ion Concentration Polarization by Bifurcated Current Path. Sci. Rep. 2017, 7, 5091. [Google Scholar] [CrossRef] [Green Version]
- Yeh, L.H.; Zhang, M.; Qian, S.; Hsu, J.P.; Tseng, S. Ion concentration polarization in polyelectrolyte-modified nanopores. J. Phys. Chem. C 2012, 116, 8672–8677. [Google Scholar] [CrossRef]
- Yeh, L.H.; Zhang, M.; Qian, S. Ion transport in a pH-regulated nanopore. Anal. Chem. 2013, 85, 7527–7534. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Gong, L.; Zhu, Y.; Gu, Y.; Han, J. Accurate Multi-Physics Numerical Analysis of Particle Preconcentration Based on Ion Concentration Polarization. Int. J. Appl. Mech. 2017, 9, 1750107. [Google Scholar] [CrossRef]
- Kim, S.; Khanwale, M.A.; Anand, R.K.; Ganapathysubramanian, B. Computational framework for resolving boundary layers in electrochemical systems using weak imposition of Dirichlet boundary conditions. Finite Elem. Anal. Des. 2022, 205, 103749. [Google Scholar] [CrossRef]
- Han, W.; Chen, X. Nano-electrokinetic ion enrichment in a micro-nanofluidic preconcentrator with nanochannel’s Cantor fractal wall structure. Appl. Nanosci. 2020, 10, 95–105. [Google Scholar] [CrossRef]
- De Valença, J.; Jõgi, M.; Wagterveld, R.M.; Karatay, E.; Wood, J.A.; Lammertink, R.G.H. Confined Electroconvective Vortices at Structured Ion Exchange Membranes. Langmuir 2018, 34, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Gong, L.; Jiang, J.; Li, Z.; Han, J. Numerical simulation of electrokinetic desalination using microporous permselective membranes. Desalination 2020, 477, 114262. [Google Scholar] [CrossRef]
- Squires, T.M.; Messinger, R.J.; Manalis, S.R. Making it stick: Convection, reaction and diffusion in surface-based biosensors. Nat. Biotechnol. 2008, 26, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; Ye, X.; Li, Z.; Han, J. Deciphering ion concentration polarization-based electrokinetic molecular concentration at the micro-nanofluidic interface: Theoretical limits and scaling laws. Nanoscale 2018, 10, 15187–15194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Do, V.Q.; Pham, S.V.; Martins, D.; Song, Y.A. A Multiwell-Based Detection Platform with Integrated PDMS Concentrators for Rapid Multiplexed Enzymatic Assays. Sci. Rep. 2018, 8, 10772. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Ouyang, W.; Li, Z.; Han, J. Direct numerical simulation of continuous lithium extraction from high Mg2+/Li+ ratio brines using microfluidic channels with ion concentration polarization. J. Membr. Sci. 2018, 556, 34–41. [Google Scholar] [CrossRef]
- Gong, L.; Li, Z.; Han, J. Numerical simulation of continuous extraction of highly concentrated Li+ from high Mg2+/Li+ ratio brines in an ion concentration polarization-based microfluidic system. Sep. Purif. Technol. 2019, 217, 174–182. [Google Scholar] [CrossRef]
- Hadidi, H.; Kamali, R. Numerical simulation of a non-equilibrium electrokinetic micro/nano fluidic mixer. J. Micromech. Microeng. 2016, 26, 35019. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Y.; Shi, P. Electrokinetic ion transport at micro–nanochannel interfaces: Applications for desalination and micromixing. Appl. Nanosci. 2020, 10, 751–766. [Google Scholar] [CrossRef]
- Peng, R.; Li, D. Effects of ionic concentration gradient on electroosmotic flow mixing in a microchannel. J. Colloid Interface Sci. 2015, 440, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Thorne, D.; Langevin, C.D.; Sukop, M.C. Addition of simultaneous heat and solute transport and variable fluid viscosity to SEAWAT. Comput. Geosci. 2006, 32, 1758–1768. [Google Scholar] [CrossRef]
- Amiri, H.A.; Asiaei, S.; Vatandoust, F. Design optimization and performance tuning of curved-DC-iDEP particle separation chips. Chem. Eng. Res. Des. 2023, 189, 652–663. [Google Scholar] [CrossRef]
- Kang, K.H.; Kang, Y.; Xuan, X.; Li, D. Continuous separation of microparticles by size with direct current-dialectrophoresis. Electrophoresis 2006, 27, 694–702. [Google Scholar] [CrossRef]
- Berzina, B.; Kim, S.; Peramune, U.; Saurabh, K.; Ganapathysubramanian, B.; Anand, R.K. Out-of-plane faradaic ion concentration polarization: Stable focusing of charged analytes at a three-dimensional porous electrode. Lab Chip 2022, 22, 573–583. [Google Scholar] [CrossRef]
- Wong, H.H.; O’Neill, B.K.; Middelberg, A.P.J. Cumulative sedimentation analysis of Escherichia coli debris size. Biotechnol. Bioeng. 1997, 55, 556–564. [Google Scholar] [CrossRef]
- Schütze, H. Coronaviruses in Aquatic Organisms. In Aquaculture Virology; Academic Press: Amsterdam, The Netherlands, 2016; pp. 327–335. ISBN 9780128017548. [Google Scholar]


| Parameter | Value | Unit | Description |
|---|---|---|---|
| 110 | [µm] | Length of the microchannel | |
| 10 | [µm] | Length of the membrane | |
| 20 | [µm] | Height of the microchannel | |
| 0.5 | [V] | Left reservoir voltage | |
| 0 | [V] | Right reservoir voltage | |
| 0–2.5 | [V] | Cross-membrane voltage | |
| 200–800 | [µm/s] | Inlet velocity | |
| 300 | [K] | Reference temperature | |
| 1000 | [kg/m3] | Fluid density | |
| 0.001 | [Pa·s] | Fluid dynamic viscosity | |
| 1.34 × 10−9 | [m2/s] | Diffusion coefficient of cation | |
| 2.03 × 10−9 | [m2/s] | Diffusion coefficient of anion | |
| +1 | [-] | Cation (Na+) covalence | |
| −1 | [-] | Anion (Cl−) covalence | |
| 1 | [mM] | Bulk concentration | |
| 2 | [mM] | Membrane concentration | |
| 40.908 | [g/mol] | Expansion coefficient | |
| 78 | [-] | Bulk relative permittivity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dezhkam, R.; Amiri, H.A.; Collins, D.J.; Miansari, M. Continuous Submicron Particle Separation Via Vortex-Enhanced Ionic Concentration Polarization: A Numerical Investigation. Micromachines 2022, 13, 2203. https://doi.org/10.3390/mi13122203
Dezhkam R, Amiri HA, Collins DJ, Miansari M. Continuous Submicron Particle Separation Via Vortex-Enhanced Ionic Concentration Polarization: A Numerical Investigation. Micromachines. 2022; 13(12):2203. https://doi.org/10.3390/mi13122203
Chicago/Turabian StyleDezhkam, Rasool, Hoseyn A. Amiri, David J. Collins, and Morteza Miansari. 2022. "Continuous Submicron Particle Separation Via Vortex-Enhanced Ionic Concentration Polarization: A Numerical Investigation" Micromachines 13, no. 12: 2203. https://doi.org/10.3390/mi13122203








